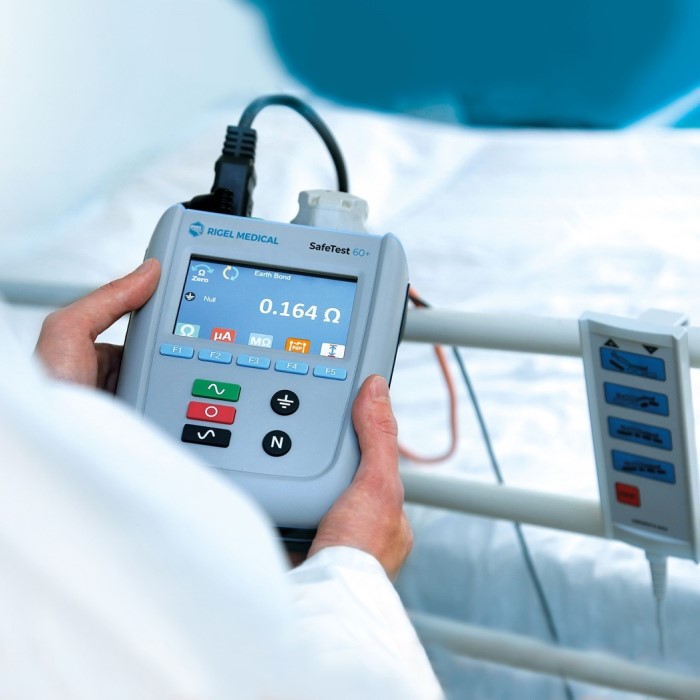
Introduction to Electrical Safety in Medical Devices
With the rise of sophisticated medical equipment, ensuring electrical safety testing devices is critical. This safety is vital not only to protect patients but also to comply with legal and regulatory standards. In this section, we focus on the importance of electrical safety standards and two central standards in the field: the IEC 60601 series and IEC 62353.

Importance of Electrical Safety Standards
Electrical safety standards are essential for safeguarding patients during the use of medical devices. These standards help to prevent electrical shocks, burns, and other potential injuries. Adhering to these standards also limits liability for manufacturers and healthcare providers, ensuring that medical devices meet stringent safety criteria.
IEC 60601 Series Standards
The IEC 60601 series is a set of international standards designed to ensure safety and effectiveness in the use of electro-medical devices. The series covers general requirements such as insulation, protection against electrical shock, and mechanical hazards.
IEC 62353 for In-Hospital Testing
IEC 62353 is the standard for routine in-hospital electrical safety testing of medical devices. This standard allows for safer ongoing use of medical equipment by setting out procedures for testing prior to use, during regular maintenance checks, and following repairs. It is intended for practical use in healthcare settings, providing a framework for ensuring devices are checked regularly and function as intended.
Global Electrical Safety Standards and Compliance
ISO and IEC Standards in Medical Device Safety
ISO and IEC set global benchmarks for medical device safety. These organizations work together to create worldwide safety standards. The IEC 60601 standard is one of the most prominent. It defines safety measures for electro-medical devices. Medical devices must meet IEC 60601 standards to ensure safety against electric shocks and other hazards.
NFPA and AAMI Standards in the United States
The United States relies on standards set by the NFPA and AAMI for medical device safety. NFPA 99, for instance, is key for health care facilities. It outlines the electrical safety testing required. AAMI sets the standard for safe current limits in electro-medical apparatus with ANSI/AAMI ES1.
UL Standards for Manufacturing
Manufacturers in the US must heed UL standards for safety. UL’s guidelines, like UL 544, set criteria for medical equipment production. These standards help ensure devices are safe before reaching hospitals or clinics.
CSA Standards in Canada
Canada adopts many IEC standards through the CSA. One main standard is CAN/CSA C22.2 NO. 60601-1-08. This ensures consistency with international safety protocols. Medical electrical devices in Canada must fulfill these requirements for sale.
Global collaboration on these standards aids in creating a uniform approach to electrical safety testing. Compliance with these regulations is vital. It ensures that medical devices are safe for patient and practitioner use worldwide.
Medical Device Classification and Types
Electrical safety testing is a central piece of the medical device certification puzzle. Tests follow strict guidelines, and devices get classified to maintain uniform standards and safety practices. Let’s dive into the classifications that stem from these guidelines.
Device Classification According to IEC Standards
Device classification under IEC standards helps ensure each device meets specific safety requirements. Three class types define the level of protection a device provides against electric shock:
- Class I devices have basic insulation and a protective earth connection.
- Class II devices have double or reinforced insulation without a protective earth.
- Class IP equipment has an internal power supply and specific safety features.
These classes serve as a foundation for ensuring patient and operator safety against electrical risks. Medical devices must pass rigorous testing to fall into one of these categories.
Type B, BF, and CF Patient Leads
Apart from device classification, medical leads that come into contact with the patient are also categorized. The types include:
- Type B Leads: Earthen Connections
- Type B leads are specifically designed to be earthed, meaning they are connected to a common ground.
- This grounding mechanism helps prevent the risk of electric shock during medical procedures.
- By establishing a safe path for electrical currents, Type B leads ensure patient safety in environments where electrical devices are used.
- Type BF Leads: Floating with Enhanced Safety
- Type BF leads, in contrast, are categorized as floating leads.
- This means they do not have a direct earth connection, providing flexibility in their use.
- Despite their floating status, Type BF leads incorporate additional safety measures such as reinforced insulation and isolation.
- These safety features enable safe contact with the patient, making Type BF leads suitable for various medical applications.
- Type CF Leads: Highest Safety Standards
- Similar to Type BF leads, Type CF leads are also floating.
- However, they are designed specifically for direct contact with the heart.
- Given their critical function, Type CF leads require the highest degree of safety and precaution during use.
- Enhanced safety features may include advanced insulation and strict testing protocols to minimize any risk of electrical interference.
- Type CF leads are essential in cardiac applications, where the safety of the patient is paramount.
Summary of Lead Types
- Type B Leads: Earthed for safety, protecting patients from electric shock.
- Type BF Leads: Floating with extra safety measures for patient contact.
- Type CF Leads: Floating, designed for heart contact, ensuring the highest safety standards.
Each type of lead is designed with specific applications and safety profiles in mind. Electrical safety testing makes sure these leads operate within set parameters to avoid harm. Manufacturers follow these classifications when developing and testing their medical devices.
Testing and Measurement of Medical Devices
Ensuring that medical devices operate safely involves a series of rigorous tests and measurements. These procedures are pivotal in detecting any electrical risks that can compromise patient care. We will discuss the key testing measures that are part of electrical safety testing.
Protective Earth Resistance
Protective earth resistance testing is essential for devices with an earth connection. It checks if the earth connection is secure. A low resistance ensures the device can safely pass fault currents away from patients.
Earth and Touch Leakage Currents
Leakage currents can pose a serious risk of electric shock. Testing for earth leakage current assesses the current that flows from the protective earth to the main supply. Touch leakage current testing evaluates the current that might pass through a person touching the device.
Patient Leakage and Auxiliary Currents
Patient leakage current tests measure unwanted currents that could pass through a patient’s body. Auxiliary currents are checked to ensure they are within safe levels. These tests are crucial for devices that come into direct contact with the patient.
Dielectric Strength/Insulation Testing
This test checks a device’s ability to operate safely under high voltage conditions. It helps to confirm that the insulation is adequate. This prevents unwanted leakage currents that could cause harm to patients and staff.
Regulations and Legal Obligations
Regulations around electrical safety testing ensure that medical devices meet legal standards. These laws protect users and keep manufacturers accountable.
CE Marking and EU Directives for Medical Devices
The CE mark is a mandatory conformity mark for products in the European Union. It shows products comply with EU safety directives. Medical devices need the CE mark to trade in the EU. Directives like the Medical Device Directive set these requirements.
Legal Consequences of Non-Compliance
Not following safety testing laws can lead to serious legal issues. This includes fines and product recalls. It can even lead to a ban on selling certain medical devices. Following regulations avoids these risks.
Documentation Requirements for Electrical Safety
Manufacturers must keep detailed records of electrical safety testing. This includes test procedures, results, and compliance with standards. These documents prove that products meet safety requirements. They must be accessible for inspection for at least 10 years after the product is sold.
Benefits of Rigorous Electrical Safety Testing
Rigorous electrical safety testing offers many benefits. It ensures devices are safe and meet high-quality standards.
Minimizing Risk of Damage and Injury
Regular electrical safety testing minimizes the risk of harm to patients and damage to property. By detecting faults early, safety is increased.
Accredited Tests and Quality Assurance
Accredited tests are done by certified labs and provide higher assurance of safety. They follow strict standards for reliable results.
Design Improvements and Technical Documentation
Through testing, design flaws can be found and improved. Documentation also gets better, helping to meet compliance and safety standards.
Electrical Safety Testing Procedures
Electrical safety testing procedures are a critical part of ensuring medical devices work safely and effectively. These procedures follow a specific sequence and adhere to international standards. Let’s delve into the aspects of these procedures.
Sequence of Testing according to IEC 62353
The IEC 62353 standard guides the testing sequence for medical devices in hospitals. This includes testing prior to patient use, during regular checks, and after repairs. Manufacturers must provide information on testing intervals based on risk and device history. Frequency is key, with life-critical devices tested every 24 months at a minimum.
- Start by checking the device visually.
- Measure the protective earth resistance.
- Evaluate earth and touch leakage currents.
- Perform patient leakage and auxiliary currents tests.
- Conclude with dielectric strength or insulation testing.
Testing equipment must meet IEC 61010-1 standards. Following these steps ensures comprehensive electrical safety assessment.
Compliance with IEC 61010-1 Standards
- Overview of IEC 61010-1 Standards
- IEC 61010-1 is an international standard that outlines safety requirements for electrical equipment used in measurement, control, and laboratory environments.
- It serves as a framework to ensure the safe operation of testing devices across various applications, particularly in medical settings.
- Safety Assurance in Testing Devices
- Compliance with IEC 61010-1 standards is crucial for maintaining the safety of testing devices.
- These standards are designed to protect both the users of testing equipment and the patients in clinical environments, minimizing the risk of electric shock or malfunction.
- Appropriateness of Measurement Tools
- Only measurement tools that have been rigorously tested and confirmed to meet IEC 61010-1 standards are deemed appropriate for use in medical procedures.
- This compliance ensures that the devices are reliable and effective in function, reinforcing their legitimacy within the medical field.
- Accuracy of Results
- Devices that comply with IEC 61010-1 standards provide accurate and reliable results.
- Maintaining high levels of accuracy is essential in medical testing, as precise measurements directly impact patient diagnosis and treatment.
- Prioritizing Patient Safety
- The primary goal of adhering to IEC 61010-1 standards is to keep patient safety at the forefront.
- By ensuring that testing devices comply with these standards, healthcare providers can minimize risks and protect patients during medical procedures.
- Safety features mandated by the standards contribute to a more secure testing environment, ultimately fostering trust in the medical testing process.
Summary of Compliance Importance
- Compliance with IEC 61010-1: Essential for safety and effectiveness in testing devices.
- Safety Assurance: Protects users and patients from risks associated with electrical equipment.
- Appropriateness: Only certified tools are suitable for medical procedures, ensuring reliability.
- Accurate Results: Compliance guarantees the precision needed in medical testing.
- Prioritizing Safety: Protecting patients enhances trust in medical practices and outcomes.
Documentation and Record-Keeping Protocols
Documentation is vital in electrical safety testing. It backs up compliance with standards and supports traceability. Records should include:
- Details of the testing group and individuals involved.
- Identification of equipment and accessories tested.
- Test types, measurements, and outcomes.
- Dates and results of visual inspections, measurements, and functional testing.
- Conclusive evaluation signed off by the evaluator.
Good record-keeping like computerized systems offers benefits. It simplifies data storage, access, review, and analysis. It ensures all device fields are standardized and comply with regulations.
Conclusion: The Path Forward in Electrical Safety Testing
Navigating electrical safety testing for medical devices is a multifaceted process. With increasing reliance on technology in healthcare, ensuring that medical equipment meets stringent safety standards is crucial. The importance of electrical safety testing cannot be overstated, as it directly correlates to patient safety and device efficacy.
By adhering to established standards, employing best practices, and staying abreast of industry trends, manufacturers can enhance their testing processes and ensure compliance. The landscape of electrical safety testing will continue to evolve, demanding ongoing vigilance and commitment to quality. As we move forward, it is essential to recognize that the safety of medical devices is fundamentally tied to the health and well-being of patients worldwide.
In conclusion, electrical safety testing remains a cornerstone of effective medical device manufacturing and deployment. Through understanding the complexities of the testing process and remaining committed to standards, the medical device industry can continue to ensure safety, reliability, and trust in healthcare technologies.